This section devoted to The History of Down syndrome reveals the field’s evolution to present day. The work of pioneers has produced vital knowledge about trisomy 21 that is advancing today’s science and improving health outcomes.
The History of Trisomy 21 Research from Description to Understanding.
The first scientific record of the genetic syndrome we now recognize as Down syndrome or Trisomy 21, was published in the London Hospital Record in 1866, authored by John Langdon Down, a British Physician. This description of the neurodevelopmental and physical changes associated with the condition was followed a decade later by a work by two Scottish physicians, John Fraser and Arthur Mitchell, who reported the early-onset of cognitive and functional decline in adults with Down syndrome. Interestingly, Fraser and Mitchell’s findings were published more than three decades prior to the description of Alzheimer’s disease in the general population. This was followed by other key research underpinning our understanding of Alzheimer’s disease pathology and pathophysiology by the study of individuals who have Down syndrome, including key papers by George Jervis in 1948, and George Glenner and Caine Wong in 1984.
It took almost another 100 years after John Langdon Down’s first description of Down syndrome, for the cause of the condition to be identified. In 1932 Charles Davenport postulated that Down syndrome and other intellectual disabilities could result from chromosomal changes but technical difficulties in the culturing of human cells and cytogenetic methods, and the disruption of World War II, delayed the identification of the genetic cause of Down syndrome.
In 1959, a team of scientists, comprising Jerome Lejeune, Raymond Turpin, and Marthe Gautier, based at the Armand-Trousseau Hospital in Paris, showed that cells from people who had Down syndrome, contained an extra copy of the smallest chromosome, now recognized as chromosome 21. Finally, the trisomy 21 aetiology of this condition is discovered, a vital point for these people and their families. This seminal result was independently replicated by Pat Jacobs and colleagues, working at the University of Edinburgh, with key clinical input from Lionel Penrose, a leading expert in Down syndrome, based at University College London.
Trisomy 21 affects the development and function of many organs of the body and, this complexity can make it challenging to understand how the extra chromosome specifically affects cognition, behaviour, physiology, and propensity to various co-occurring health conditions. Advances in the understanding of this complexity was significantly accelerated by the identification of the first viable mouse model of Down syndrome – the Ts65Dn – by Muriel Davidson and colleagues working at the Jackson Laboratory, in 1990. This and subsequent rodent models of Trisomy 21 have led to a step-change in the understanding of the condition. Similarly, the completion of the sequence of chromosome 21 in 2000 by an international collaborative team, established by the Trisomy 21 research community in 1996, was a landmark achievement in our understanding of Down syndrome.
In recent years, we have witnessed considerable expansion of scientific knowledge in both preclinical and clinical realms of Down syndrome research. In addition to the broad availability of rodent models of Trisomy 21, preclinical advances have been primarily driven by technical advances in areas such as in vitro cell and tissue modelling, site-directed mutagenesis, chromosomal engineering, and next-generation sequencing. Significant clinical advances have also been made in several areas of Down syndrome, however, advances toward a comprehensive characterization of the neurodevelopmental and clinical phenotypes and the validation of various biomarkers of Alzheimer’s disease arguably have had the greatest impact in evidence-based clinical practice and the design of therapeutic trials.
As T21RS celebrates its 10th anniversary, we are proud to continue to serve as a catalyst to the expansion of research in Down syndrome. In addition, the continued addition of young investigators to our ranks make us optimistic about a future in which research will play a progressively more important role in the improvement of quality of life for those with Down syndrome and their families.
Charles Epstein, a pioneer in Down syndrome research
“The science is finally catching up to the clinic.”
Dr. Charles Epstein, a UC San Francisco medical geneticist who studied Down syndrome and pioneered genetic counseling for families with affected children. His major research interests were in the genetics of early embryonic development, the pathogenesis of Down syndrome, genetic approaches to the study of free radical defense mechanisms, and aging. He hypothesized in the early 1970s that the abnormalities found in Down syndrome were due to an increased dosage of critical genes on chromosome 21. His dosage hypothesis was not the favored model at the time but has proven correct.

Sago H; Carlson EJ; Smith DJ; Rubin EM; Crnic LS; Huang TT; Epstein CJ. 2000. Genetic dissection of region associated with behavioral abnormalities in mouse models for Down syndrome. Pediatr Res 48(5):606-13.
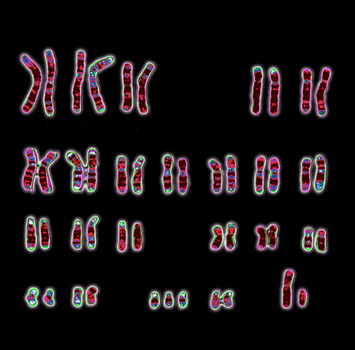
He investigated one of the first genes identified on human chromosome 21, the interferon-alpha receptor gene (IFNAR1) and also the role of SOD1 and other homologs in free radical metabolism in aging. He decided to use the mouse as a model when starting his own lab to study early preimplantation mouse development and made important discoveries regarding the timing of initiation of X chromosome inactivation. These studies convinced him of the power of using a mammalian model such as the mouse for studying human genetic diseases, and several created mouse models relevant to the field.
He was also one of the first to formulate the conception that birth defects are caused by mutations in genes that act as pathways—inborn errors of development—similar to biochemical pathways. These examples—the study of human diseases such as Down syndrome with mouse models and the concept of inborn errors of development—also illustrate the integration of the basic principles of human genetic diseases with clinical genetics that was a hallmark of Charle’s contribution to the field and was a major motivating force in his research. He was the recipient of the two major awards of The American Society of Human Genetics (ASHG) in addition to serving as its President (1996) and as the editor of the Society’s journal (1987–1993). As he noted in his McKusick Leadership Award address, “The science is finally catching up to the clinic.”
Charles Epstein died on February 15, 2011, at the age of 77, after a year-long battle with pancreatic cancer.
Letter in honor to Marthe Gautier, honorary member of T21RS.
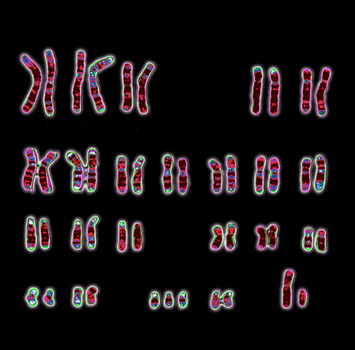
He investigated one of the first genes identified on human chromosome 21, the interferon-alpha receptor gene (IFNAR1) and also the role of SOD1 and other homologs in free radical metabolism in aging. He decided to use the mouse as a model when starting his own lab to study early preimplantation mouse development and made important discoveries regarding the timing of initiation of X chromosome inactivation. These studies convinced him of the power of using a mammalian model such as the mouse for studying human genetic diseases, and several created mouse models relevant to the field.
He was also one of the first to formulate the conception that birth defects are caused by mutations in genes that act as pathways—inborn errors of development—similar to biochemical pathways. These examples—the study of human diseases such as Down syndrome with mouse models and the concept of inborn errors of development—also illustrate the integration of the basic principles of human genetic diseases with clinical genetics that was a hallmark of Charle’s contribution to the field and was a major motivating force in his research. He was the recipient of the two major awards of The American Society of Human Genetics (ASHG) in addition to serving as its President (1996) and as the editor of the Society’s journal (1987–1993). As he noted in his McKusick Leadership Award address, “The science is finally catching up to the clinic.”
Charles Epstein died on February 15, 2011, at the age of 77, after a year-long battle with pancreatic cancer.

Dr. Marthe Gautier passed away the 30th of April 2022 at the age of 96. In team collaboration with Dr. Jerome Lejeune and Dr. Raymond Turpin, Dr. Gautier brought expertise to show for the first time the presence of a supernumerary chromosome in the cells from 9 individuals with “mongoloid” features as it was called in the 50ths. This discovery was the result of a year she spent in Harvard (1955-1956) to learn new technics of human cell culture (she co-authored in 1958 a paper in AMA Arch Pathol. on “Cells of human heart and aorta grown in tissue culture”), and of two years to implement these new techniques at the Trousseau hospital in the team of Dr. Turpin who gave her a lab to “count the number of chromosomes in cells from individuals with Down syndrome”. This team discovery was confirmed by a paper published by Dr. Patricia Jacob in the same year. This signified the beginning of a new era studying the genetics of chromosomal defects and represented a big step for the diagnosis of individuals with Down syndrome.
